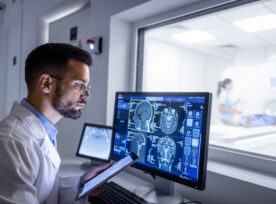
In 2025, the medical device industry trends are not just shaping the future—they’re redefining the present. As technology advances at an unprecedented pace, regulatory landscapes evolve, and patient expectations rise, the industry stands at a pivotal juncture. Embracing these trends offers a pathway to innovation, market expansion, and enhanced patient outcomes. However, success requires strategic […]
Posts Tagged ‘Compliant Medical Devices’