Last week we covered dashboards in Oracle’s Argus Safety. Today we’ll talk about Worklists. Worklists in the drug safety and pharmacovigilance system can be seen as the end-users task list for the day. While there are many Worklists to help organize a company’s users, today we will focus on two of the main ones, which are tightly integrated to the configured workflow of Argus Safety.
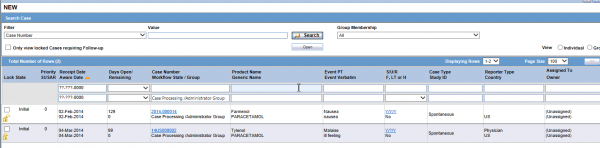
The Worklist New is an interface within Argus Safety that allows you to visualize all of the adverse event cases that have not yet been assigned to an individual or workflow group for which the user has access. Each time a case is passed along a workflow step, it returns to the Worklist New area and waits for acceptance by a user or assignment by a workflow manager.
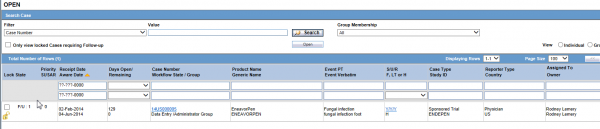
The Worklist Open is very similar to Worklist New, except that it shows all of the cases that have already been assigned to either a user or group. This can empower a company to continue with business as usual if someone is out sick for the day and someone needs to take over their cases, as long as they are both part of the same workflow group. This can help eliminate potential regulatory timeline delays.
Stay tuned for our next Argus Safety feature-related post. To read past posts in this series, click here.