In April 2014, I read a startling article that the United States was importing salt water, saline, from Norway due to a shortage in the United States1. Bags of saline solution are one of the most common items used in modern healthcare and that is why it is amazing that American doctors have been facing a bizarre IV saline shortage that forces the import of heavily, unwieldy bags of salt water from overseas. As a result, hospitals, and especially cancer centers, have been keeping strict inventories of how many bags are on hand and struggling to avoid rationing their use. This turn of events led to a deeper look at the problems caused by drug shortages for healthcare organizations and developing a business intelligence blueprint to help manage shortages more effectively.
The IMS Institute for Healthcare Informatics, in their report Drug Shortages: A closer look at products, suppliers and volume volatility, identified 6 key insights:
- The drug shortage problem is highly concentrated to generic injectables or five disease areas including oncology and cardiovascular diseases.
- The supply of drugs on the shortages list has been stable or increased overall in the past five years.
- There is significant volatility in the suppliers of the drugs, not the total volume supplied.
- The recent volatility is a new trend compared to previous years.
- The number of suppliers has fluctuated and may be one reason for the volatility.
- Some states are feeling the drug shortage more acutely than others.
After studying a research survey from the November/December 2013 issue of the Journal of Managed Care Pharmacy entitled “Effects on Patient Care Caused by Drug Shortages: A Survey”, several key aspects of the blueprint for managing drug shortages began to emerge:
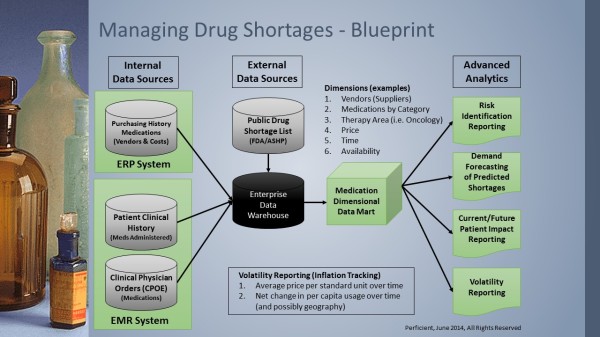
- Purchasing history of medications including vendors, costs and availability, typically from an Enterprise Resource Planning (ERP) system, needed to be integrated with physician orders for medications and patient history of medications administered, generally available from an Electronic Medical Record (EMR) system.
- External data from a public drug shortage list compiled and maintained by the Food and Drug
Administration (FDA) and American Society of Health-System Pharmacists (ASHP) could help identify key medications to monitor.
- The internal data sources, the ERP and EMR information, and external data source of drug shortages should be normalized into the healthcare organization’s enterprise data warehouse to allow tracking of patient safety, adverse events, delayed patient care and other impacts of the drug shortages.
- A dimensional data mart for medications would be created over the enterprise data warehouse to allow advanced analytics especially for viewing trends and predicting future shortages or patient impacts. A few of the key dimensions would be medication vendors, categories of medications, therapy areas impacted by shortages, price or cost, inventory availability and, of course, time.
- Reporting from the dimensional data mart would provide a healthcare organization with an early warning system for drug shortages. Some of the key reports would be risk identification reporting which examines the vendors, how many drugs are provided by key vendors and the financial expenditures with those vendors. Demand forecasting reporting would look at the combination of purchased medication history, past patient clinical history of medications administered and current physician medication orders to predict potential shortages for risk mitigation. Volatility reporting could track average medication price over time to provide visibility of inflation or sudden increases in per capita uses of key drugs. Most importantly, current and future patient impact report would predict potential delays in treatment, identify alternatives for timely treatment and help minimize patient impacts.
This blueprint for managing drug shortages can help hospital administrators, pharmacy managers and physicians take a closer look at the products, the suppliers or vendors, and the volume or price volatility in a rapidly changing marketplace. The number of drug companies supplying these key products, like saline IV bags, has fluctuated over the last five years with an increasing number of companies no longer supplying the products on the FDA/ASHP shortages list. With medication suppliers citing production-related issues and increased demand as the top reasons for shortages, now is the time to act to create these early warning systems and protect patient safety.
- “We’re Importing Salt Water from Norway“
- “Effects on Patient Care Caused by Drug Shortages: A Survey“, Journal of Managed Care Pharmacy JMCP November/December 2013 Vol. 19, No. 9
- Drug Shortages: A closer look at products, suppliers and volume volatility. Report by the IMS Institute for Healthcare Informatics, November 2011.