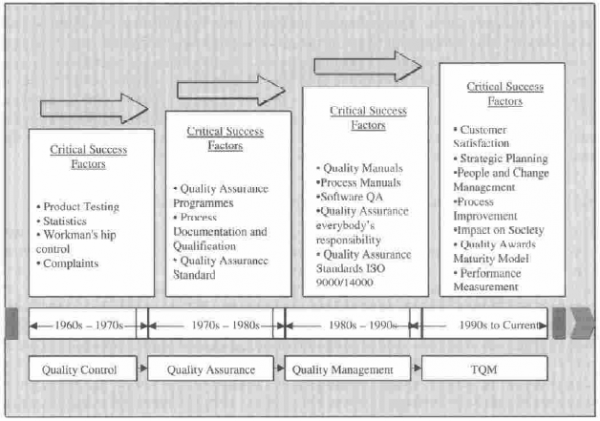
Over time, the concept of Quality Control has evolved to become Total Quality Management (TQM). According to Wikipedia, the concept of TQM “…consists of organization-wide efforts to install and make a permanent climate in which an organization continuously improves its ability to deliver high-quality products and services to customers.” Since a CAPA system is part of TQM, it needs to provide functionality that supports compliance with internal and external quality governance.
Over the next 6 blog posts, we’ll specifically address how Oracle’s drug safety and pharmacovigilance system, Argus Safety, can help you comply with the following CAPA rules:
- System must provide workflow configuration to handle internal quality control, quality assurance and/or quality management issues
- System must be able to uniquely identify complaint cases
- System must be able to attach supportive information to the case data
- System must allow the recording of issues and track their resolution to completion either in the form of Corrective, Preventative or both action types
- System must produce ad-hoc descriptive statistical reports on quality data
- System must comply to 21 CFR Part 11
Evolution from Quality Control to Total Quality Management
To read other posts in our “Argus Safety for CAPAs” series, click here.